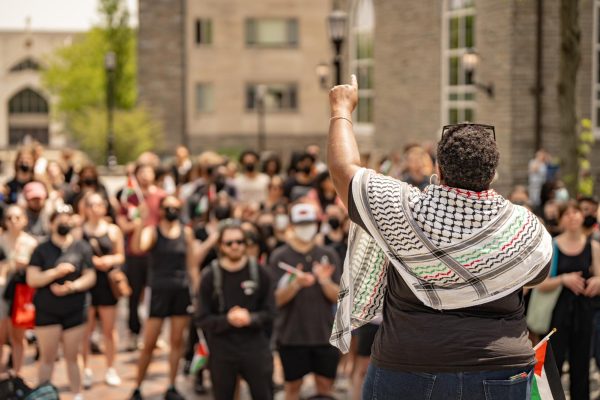FDA Authorizes Johnson & Johnson Covid-19 Vaccine
March 7, 2021
The Food and Drug Administration (FDA) granted emergency authorization for the Johnson & Johnson COVID-19 vaccine on Saturday, giving the go ahead for the new vaccine’s rapid distribution in the United States.
The Johnson & Johnson vaccine is the third one developed in the last year to receive FDA approval, joining the Pfizer and Moderna vaccines in the fight against the still rampant COVID-19 pandemic in the United States.
President Joe Biden expressed his excitement with the vaccine authorization in a press briefing on Saturday, declaring that “thanks to the brilliance of our scientists, the resilience of our people, and the eagerness of Americans in every community to protect themselves and their loved ones by getting vaccinated, we are moving in the right direction.”
According to the CDC, as of Feb. 28, 14.6% of the U.S. population has received at least one dose of the COVID-19 vaccine, and 7.1% of the population is fully vaccinated. The addition of the Johnson & Johnson vaccine to the distribution supply will allow that number to increase quickly.
The clinical trial for the Johnson & Johnson vaccine was vast, involving almost 45,000 participants in eight countries across three continents. According to a report by the FDA, the Johnson & Johnson vaccine has a 66.9% efficacy rate, meaning it is less effective than the vaccines currently in distribution throughout the nation. The Pfizer and Moderna vaccines each have an efficacy rate around 95%.
While the vaccine is still highly effective, U.S. health officials are concerned that the Johnson & Johnson vaccine’s lower efficacy rate will lead Americans to believe it to be a less favorable option than the Pfizer or Moderna shots. In an interview following the vaccine’s approval, Dr. Anthony Fauci urged Americans to overlook the differences in efficacy rates and “accept the fact that now you have three highly effective vaccines. Period.”
Unlike the Pfizer and Moderna vaccines, the Johnson & Johnson vaccine is one dose instead of two, allowing for more efficient mass vaccination. The Johnson & Johnson vaccine also poses fewer storage obstacles than its counterparts, which were developed using mRNA technology that requires them to be kept at freezing temperatures. The Johnson & Johnson vaccine can be kept at normal refrigeration temperatures for up to three months, making it easier to store and distribute.
Fauci expressed his excitement about the new vaccine, stating during a White House briefing that “we now have three highly effective vaccines. Importantly, each of them are very effective against severe disease, and virtually all of them say that you look at the data, and it’s clear that you get essentially no hospitalizations or deaths in any.”
While the clinical trials of the Johnson & Johnson vaccine are promising, the firm has fallen short of the production goals set in its $1 billion federal contract. While only four million doses are
prepared for shipment compared to the projected 12 million, Johnson & Johnson still aims to fulfill the planned 100 million doses by the end of June. Shipments will begin as soon as Monday.
Despite the production issues facing the vaccine rollout, President Biden took an optimistic tone in a press briefing Thursday, asserting that “we have a plan to roll it out as quickly as Johnson & Johnson can make it. We’ll use every conceivable way to expand manufacturing of the vaccine, and we’ll make even more rapid progress on overall vaccines in March.”
This optimistic news comes just as the United States reaches the devastating milestone of 500,000 COVID-19 deaths, more than any other country in the world. The virus has become a leading cause of death in the U.S. and has resulted in a decrease in the national life expectancy by one full year.